Dipole-dipole forces between water molecules. So we can see that the dispersion bond is the weakest intermolecular force and the Ion-ion force is the most potent force.
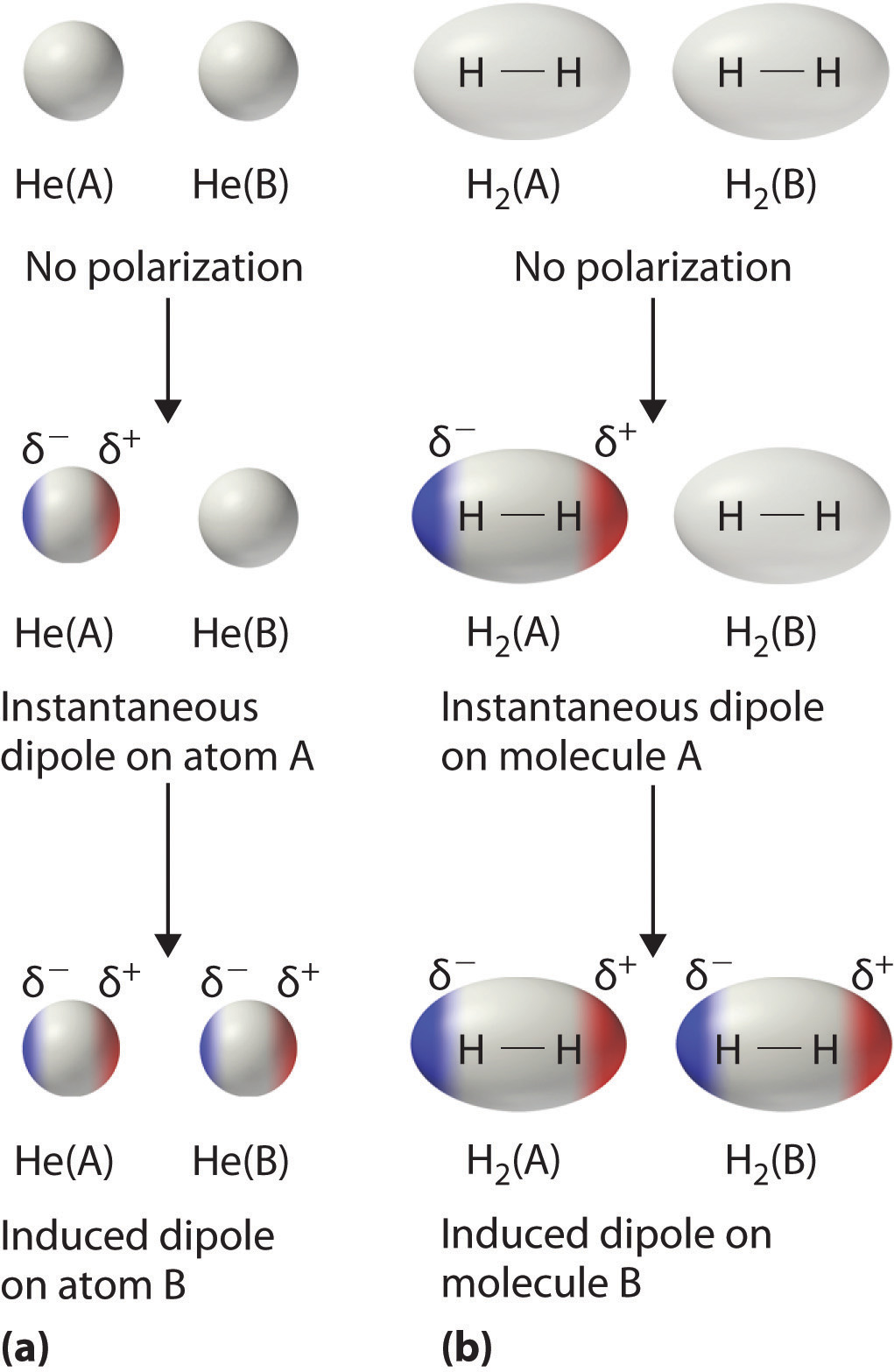
12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts
Since CO_2 is made of one carbon and 2 oxygen and both carbon and oxygen are non-metals it also have covalent bonds.

. Molecule-ion attractions between water molecules. The capability of a molecule to become polar is called polarizability of molecules. Both of these forces are due to momentarily dipole formation.
The dipole interactions are stronger than the dispersion forces because the oxygen will almost always have slightly more electrons than the. The London dispersion bond is weaker than the dipole-dipole bond which is more fragile than H-bonding which is in turn weaker than the Ion-ion bond. Van der Waals forces can be classified as weak London dispersion Forces and stronger dipole-dipole forces.
Dipole-dipole London dispersion also known as Van der Waals interactions hydrogen bonding and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds. London dispersion forces are the weakest type of non-covalent interaction. Dispersion Forces Dipole-dipole Hydrogen bonds Dispersion forces are.
For extra information there are 3 types of intermolecular forces. They are also known as induced dipole-induced dipole interactions and present between all molecules even those which. Hydrogen bonds between water molecules.
The displacement of electrons causes a nonpolar molecule to be a polar molecule. What force explains the ability for water molecules to dissolve ionic compounds. All of them are electrostatic interactions meaning that they all occur as a result of the attraction between opposite charges and which of these forces is present or.
Dispersion Forces CO_2 has dispersion forces or van der waals forces as its only intermolecular force. In organic molecules however the multitude of contacts can lead to larger contributions particularly in the presence of heteroatoms. London dispersion forces between water molecules.
Now that we have answered the question of what dispersion forces are and understood the London forces. As we move from top to bottom in a group.

10 Liquids Solids And Intermolecular Forces Chemistry Etsy In 2021 Chemistry Notes Intermolecular Force Chemistry

Why Are Dipole Dipole Forces Stronger Than Dispersion Lisbdnet Com
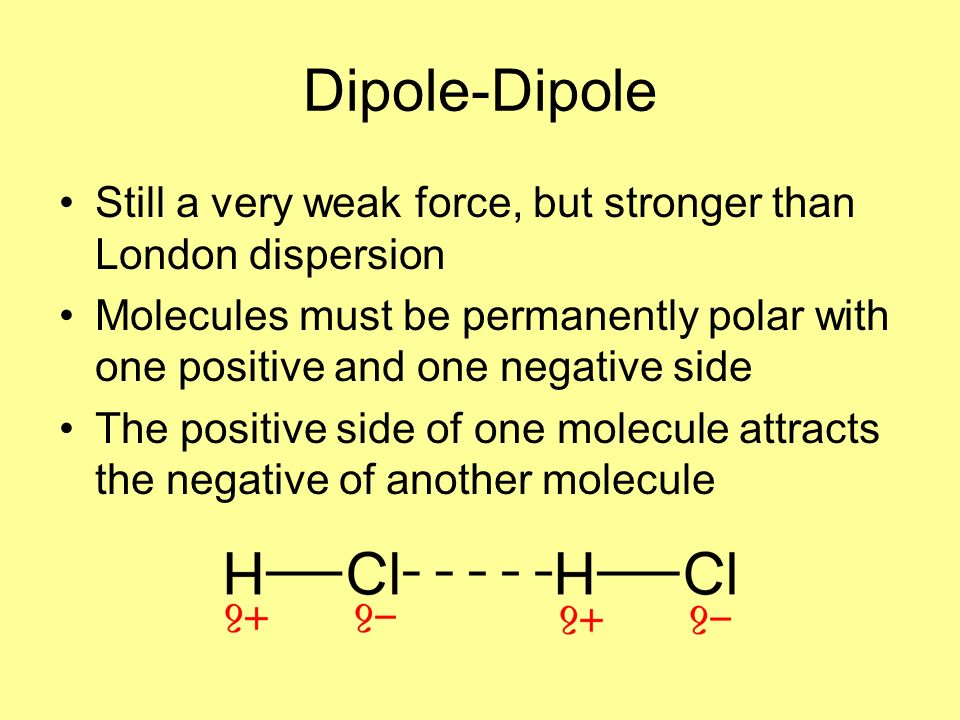
Intermolecular Forces Of Attraction Imfs Ppt Video Online Download

10 Liquids Solids And Intermolecular Forces Chemistry Etsy In 2022 Chemistry Notes Intermolecular Force Chemistry
Difference Between Dipole Dipole And London Dispersion Forces Pediaa Com
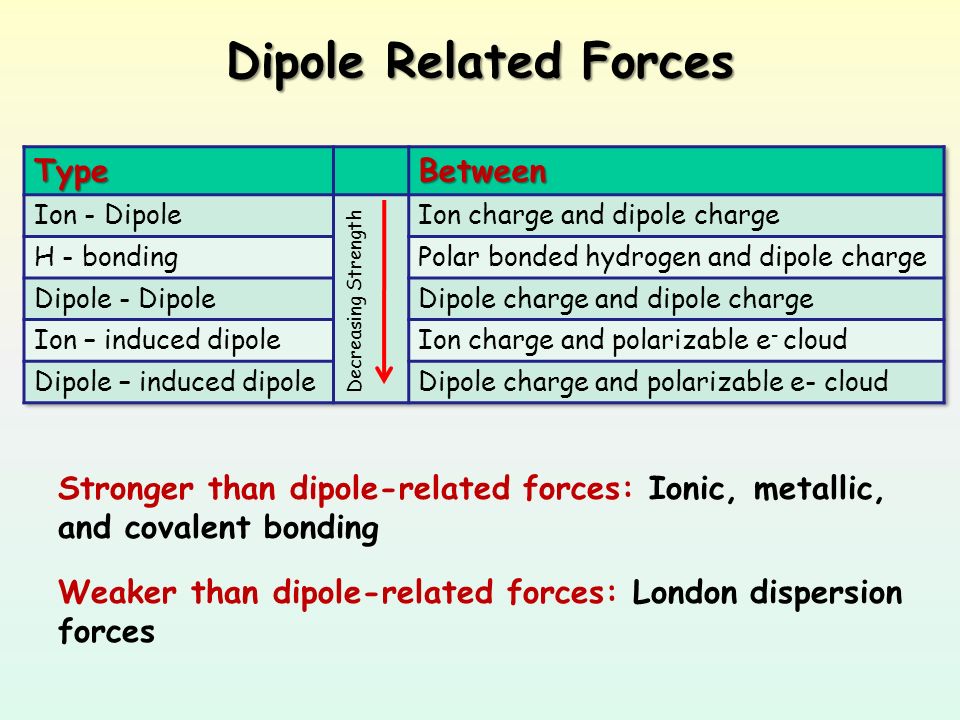
Intermolecular Forces Relative Magnitudes Of Forces The Types Of Bonding Forces Vary In Their Strength As Measured By Average Bond Energy Covalent Bonds Ppt Download
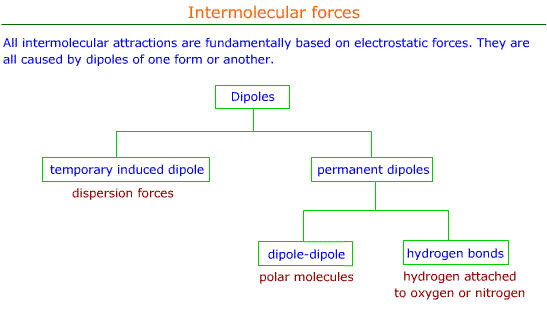
Structure And Bonding 2 41 Dispersion Forces
In What Case Are London Forces Stronger Than Dipole Forces Quora

0 Comments